|
|
|
|
|
 |
|
Korea Food & Drug Administration
|
|
|
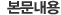 |
Korea Food & Drug Administration
#5, Nokbun-dong, Eunpyung-Gu, Seoul, Korea, Tel: 82-2-380-1659, Fax: 82-2-383-2870
Certificate of Good Manufacturing Practice
Representative
Name Of
Manufacturer
Address(Plant)
Registered
Production
Manager
Registered
Quality Control
Manager
Approved Dosage Forms
Approval Date
It is hereby certified that the above manufacturing plant in which the products are produced is subject to inspections at suitable intervals and the manufacturer conforms to GMP(Good Manufacturing Practice) as recommended by WHO.
Director General
Pharmaceutical Safety Bureau
Korea Food and Drug Administration |
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
